Abstract
Introduction
Light chain (AL) amyloidosis is caused by progressive organ dysfunction due to the deposition of structurally abnormal monoclonal light chains (LC) as amyloid fibrils. Serial free light chain measurement is the cornerstone of AL diagnosis but is limited by current methods which measure normal polyclonal and abnormal monoclonal LCs. Since each individual monoclonal LC has a unique amino acid sequence, mass spectrometry (MS) has the ability to detect serum monoclonal LCs.
We report a novel MS method for monoclonal LC detection in AL amyloidosis.
Methods
Twenty patients with systemic AL amyloidosis, diagnosed and treated at the UK National Amyloidosis Centre (UK-NAC), were randomly selected. This included 16 newly diagnosed patients, 2 patients in a complete haematological remission (CR) post-treatment and 2 patients with no amyloidosis (acting as negative controls). All patients had detailed baseline assessments of organ function and serum FLC measurements. Organ involvement was defined according to the international amyloidosis consensus criteria. Magnetic microparticles were covalently coated with modified polyclonal sheep antibodies monospecific for free kappa light chains (anti-free k) and free lambda light chains (anti-free l). The microparticles were incubated with patient sera, washed and treated with acetic acid (5% v/v) containing TCEP (20 mM) in order to elute free light chains in monomeric form. Mass spectra were acquired on a Microflex LT/SH smart matrix-assisted laser desorption ionization time-of-flight mass spectrometer (MALDI-TOF-MS; Bruker, GmbH).
Results
The median number of organs involved was 2 (range 1-4); cardiac involvement was most common in 70% (14/20) patients, followed by renal 40% (8/20), autonomic and soft tissue in 15% (3/20) and peripheral nerve involvement in 5% (1/20). No patients had liver involvement. The median N-terminal pro b-type natriuretic peptide (NT-proBNP) and cardiac troponin T were 3761.5 (range 245-25348 ng/L) and 35.5 (8-170 ng/L) respectively. The median presenting serum albumin was 37 (19-45 g/L) and eGFR 62 (10-100 mls/min). The amyloid deposits were typed as AL lambda in 14(70%), kappa in 2(10%) patients and amyloid of uncertain type in two cases (10%). The median involved FLC was kappa 76 mg/L (range 71-440) and lambda 185 mg/L (44-1023), with difference involved to uninvolved (dFLC) 118 mg/L (33-1015). An intact monoclonal protein was present in 70% (14/20).
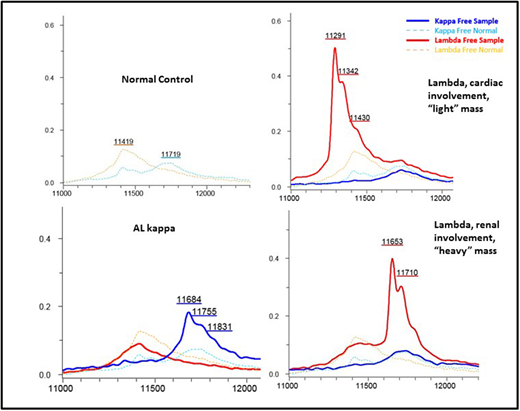
In all cases, MS correctly identified the presence and type of monoclonal LC, identifying a monoclonal lambda in 14/14 (100%) and a monoclonal kappa in 2/2 (100%); the two patients where a clear monoclonal component could not be identified were patients whose amyloid fibrils failed to be typed by immunohistochemistry or MS. The assay also confirmed normal polyclonal expression of both kappa and lambda LCs in the two control samples. In the two lambda patients in a CR, the MS method identified monoclonal lambda LC expression; in one of these patients next-generation sequencing (NGS) confirmed minimal residual disease (MRD). A clear shift in the LC mass spectra was seen relating to the specific pattern of organ involvement: in patients with renal amyloid the monoclonal LC predominantly displayed a "heavy" molecular mass, (with a mean molecular mass of 11596+/- 436 daltons), whereas in patients with cardiac involvement the monoclonal LC mainly exhibited a "light" mass (with a mean molecular mass of 11443 +/- 102 daltons).
Conclusion
This small study shows that monoclonal light chains can be accurately detected by MS and be concordant to the tissue amyloid type. A monoclonal LC was detected in 2 patients in serological "CR" - in one case presence of persistent disease was demonstrated by NGS-MRD. Even in this small sample size, there appears to be a marked difference in LC mass for patients with cardiac ("light" LC) vs. renal ("heavy" LC) involvement, raising the interesting possibility that "heavy" LC are trapped by the glomerulus causing renal AL but are unable to penetrate the tight cardiac capillary gap junctions, and vice versa. The unique molecular location of LC on MS offers the possibility of exploiting this technique as a tool to detect amyloidogenic FLC and potentially predict organ involvement in patients with gammopathies. We plan to expand this study to a large cohort of patients to confirm these findings and assess the impact on survival and organ response outcomes.
Wechalekar:Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal